What Happens at the Triple Point of Water Apex
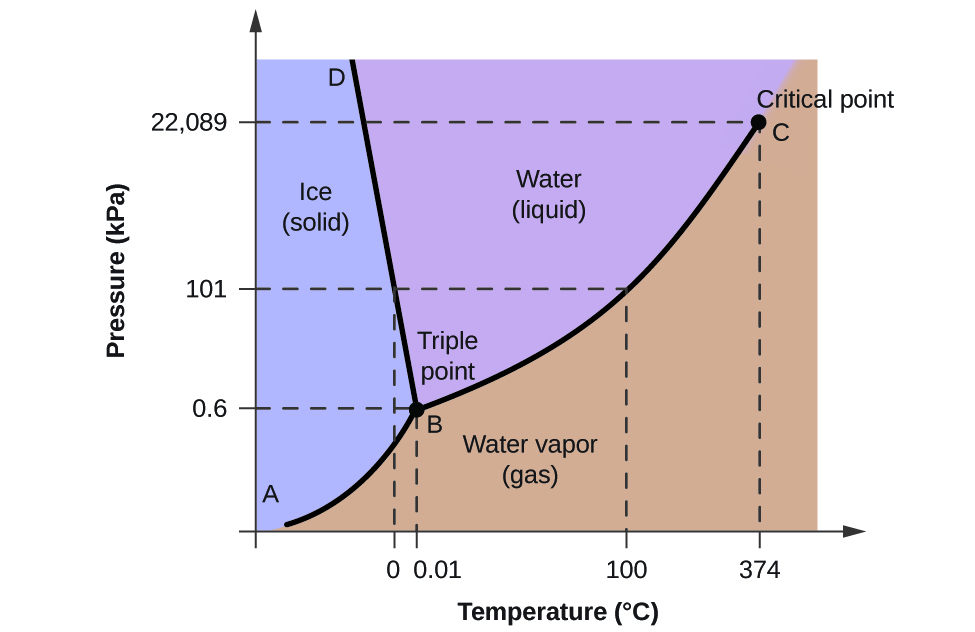
The triple point of water is the point at which water can exist at all three states of solid liquid and gas at the same time. The triple point of water is a fixed quantity used to define other triple point values and the kelvin unit of temperature.
At pressures below the triple point as in outer space solid ice when heated at constant pressure is converted directly into water vapor in a process known as sublimation.
. Liquid solid and gas coexist in a thermodynamic equilibrium. The triple point is the only condition in which all three phases can. Interestingly small changes in the temperature or pressure at this point can cause the whole of the substance to convert to one.
Solid ice liquid water and gas water vapour. This agreement also sets the size of the kelvin as 127316 of the difference between the triple-point temperature of water and absolute zero. Looking at it from the view of molecular interactions at that.
The triple point of a phase diagram is the location where the solid liquid and gas phases meet. This agreement also sets the size of the kelvin as 127316 of the difference between the triple-point temperature of water and absolute zero. This is equivalent to 001oC and 3202oF and thus we use T3 to help set the freezing point of water.
The triple point of water T 3 27316 K is the standard fixed-point temperature for the calibration of thermometers. Correct option is A Triple point of water is defined as the temperature and pressure at which liquid water solid ice and water vapour can coexist in a stable equilibrium. So that makes the question a little tricky.
Increasing the pressure further would result in only water liquid being in your container. The phase diagram of water is a pressure-temperature diagram for water that shows how all three. This temperature is 001C.
What is the triple. Above the triple point solid ice when heated at constant pressure first melts to form liquid. The triple point of pure water is at 001C 27316K 3201F and 458 mm 6112Pa of mercury and is used to calibrate thermometers.
This is the triple point of water as shared in this silent video by the UC Santa Cruz Physics Demonstration Lab at UCSC Physics. The triple point represents the combination of pressure and temperature that facilitates all phases of matter at equilibrium. At the triple point the liquid solid and gas have the same G per molecule.
Exploring Waters Triple Point. The combination of temperature and pressure that allows water to exist in its three states simultaneously is achieved at 27316 K and a partial vapor pressure of 611657 pascals. At that point it is possible to change all of the substance to ice water or vapour by making infinitesimally small changes in pressure and temperature.
Even at an ordinary two-phase transition point its sort of an accident of the prior history how much thermal energy or. When the pressure and temperature of ice are above the triple point pressure and the temperature of water is increased melting occurs. Simply put the triple point of water is the only temperature at which water can exist in all three states of matter.
When ice is exposed to moist air with a partial pressure of water below its triple point pressure heating of the ice will result in a phase change from ice directly to vapor without first going through the liquid phase. With most substances the temperature and pressure related to the triple point lie below standard temperature and pressure and the pressure for the critical point lies above standard pressure. The triple point of water is zero degree Celsius this isbecause1zero degree Celsius is the melting point of ice2water changes from liquid to solid in zero degree Celsius.
Absolute zero is defined as being equivalent to that of 0 Kelvin which is -27315oC and and -45967oF and this is a standard that is accepted internationally. Triple point of water is 273 K temperature and 046 cm of mercury pressure. Water reaches its triple point at just above freezing 01 C and at a pressure of 0006 atm.
At the triple point of water all the three phases of water ie. Therefore at standard pressure as temperature increases most. The gasliquidsolid triple point of water corresponds to the minimum pressure at which liquid water can exist.
The triple point of water is the point at which water can exist at all three states of solid liquid and gas at the same time. The triple point of water is important enough to merit its own line. The triple point occurs where the solid liquid and gas transition curves meet.
The general rule is that at fixed temperature and pressure the material will end up in a form which gives it the lowest free energy G. As at triple point of water both solid ice and vapour state are present so we can say that boiling point and freezing point become same. It is the temperature and pressure at which a given substance can assume any of the 3 usual phases.
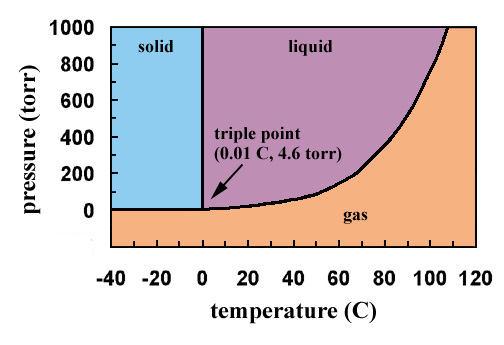
If you repeated the experiment at the triple point temperature at low pressures you would first have water vapour but at a certain pressure you would find that all three phases of water solid liquid and vapour are coexist together in equilibrium. The triple point for water is at 001 degree Celsius at 456 mm Hg. The triple point is the only condition in which all three phases can coexist and is unique for every material.
The triple point of water occurs at exactly 27316 degrees Kelvin 001 degrees Celsius and at a partial vapour pressure of 61173 pascals. Note the triple point may include more than one solid phase if a specific substance has polymorphs. The triple point occurs where the solid liquid and gas transition curves meet.
The triple point of water T 3 27316 K is the standard fixed-point temperature for the calibration of thermometers. It is called triple point because at a particular temperature and pressure for particular substances solid liquid and vapor phases coexist in equilibrium or we can say that the point where liquid becomes stable is called the triple point. With the pressure lowered the liquid water begins to boil while ice cubes remain.
Currently only transportable to Thimann Lecture halls.

The Triple Point Of Water Co Existence Of Solid Liquid And Gas Thermodynamics Point Dewar

Invaderxan Phase Diagram For Water The Triple Point Is The Point At Which The Three Phases So Physics And Mathematics Chemistry Classroom Chemistry Lessons
0 Response to "What Happens at the Triple Point of Water Apex"
Post a Comment